Metal-Ligand Multiple Bonds
Metal-ligand multiple bonds are associated with diverse reactivity patterns, including transformations that are applicable to atom-economical catalysis, e.g. atom/group transfer reactions. The redox-richness of base metal complexes (e.g. Mn, Fe, Co), coupled with their ability to access multiple spin states, is expected to afford metal-ligand multiple bond complexes with unusual electronic structures and reactivity patterns.
We target metal-ligand multiple bonds in base metal complexes that are supported by strongly-donating, multidentate carbene ligands. These modular ligands provide a high degree steric and electronic flexibility, and importantly create an ideal framework for stabilizing reactive metal-ligand multiple bonds, including for atomic ligands.
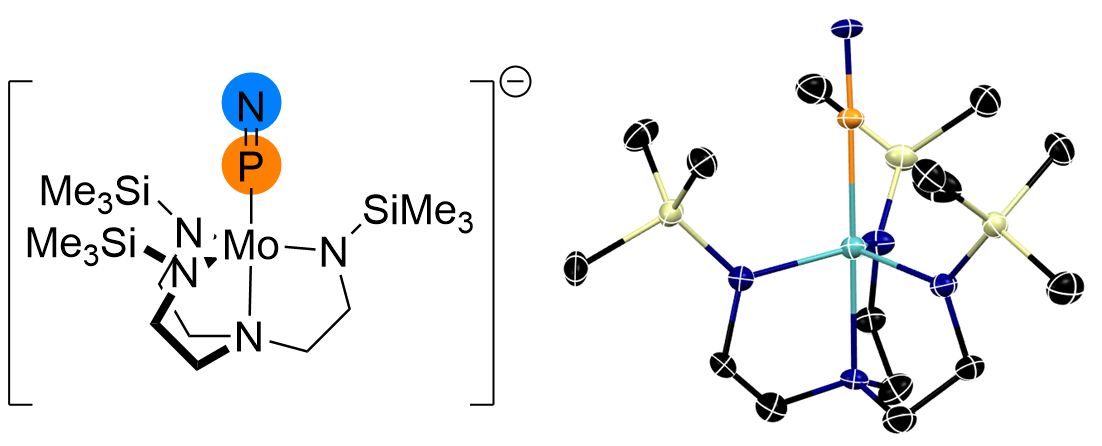
Our group has pioneered the chemistry of iron(IV) nitrido complexes supported by bulky tris(carbene)borate ligands, and have recently extended these to other single atom ligands. These complexes are reactive in multielectron reactions, including nitrogen atom transfer to a range of hydrocarbon substrates. We have also used these complexes as synthons in the assembly of new ligands, including the first structurally-characterized example of the interstellar gas, P≡N.
Spin as a Design Element in Organometallic Catalysis
Many base metal complexes can access multiple spin states, which have different reaction landscapes. While this diversity of electronic states has traditionally been viewed as a significant challenge for catalyst design, it also provides an opportunity to create catalysts that can modulate access to these states, providing a new mechanism for tuning reactivity.
The goal of this project is to develop new low-valent base metal complexes for application in organometallic catalysis, where the spin state dynamics of species in the catalytic cycle are a key design principle. A critical aspect of this work is the development of new ligand frameworks that provide access to multiple spin states in coordinatively unsaturated environments. In an initial demonstration of the potential of this strategy, we have discovered that the spin state dynamics of an alkene isomerization catalyst confers resistence to poisoning by strong σ-bases. Experimental and computational mechanistic investigations reveal that access to the catalytically active low spin state is gated by the alkene substrate, and that poisoning ligands only interact weakly with the high spin ground state.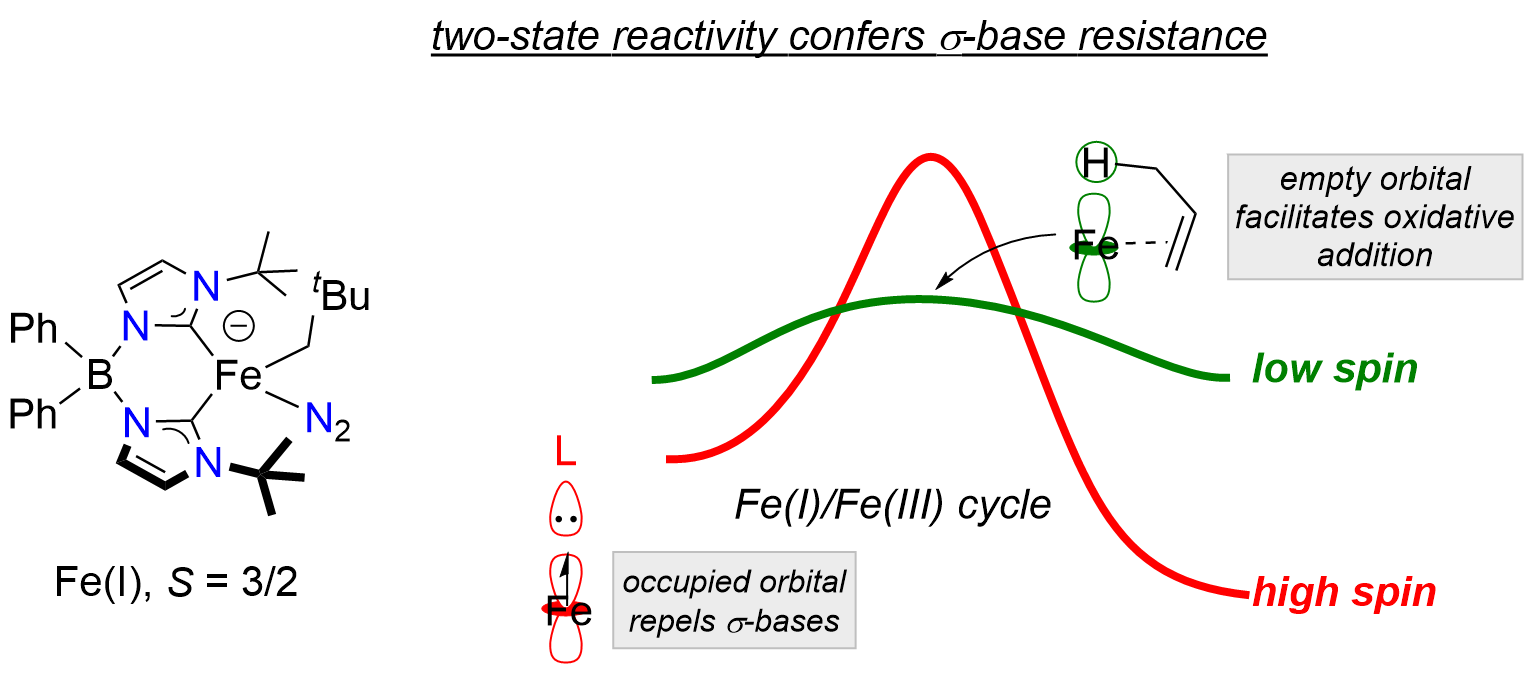
Nitrogen Cycle Electrocatalysis
Due to the massive influx of Haber–Bosch derived ammonia fertilizers, anthropological sources now dominate the production of bioavailable nitrogen. Nitrification converts more than half of agriculturally applied NH3 to nitrate and other oxidized nitrogen species, which washes downstream and oversupplies nutrients to waterborne algae and creates aquatic “dead zones”.
We aim to develop molecular electrocatalysts for aqueous nitrate reduction at low overpotential, along with high substrate and product selectivity, and at reasonable turnover frequencies. While nitrate reduction is thermodynamically favorable, its weak binding, kinetic inertness and complex redox chemistry are significant challenges to the development of aqueous electrocatalysts.
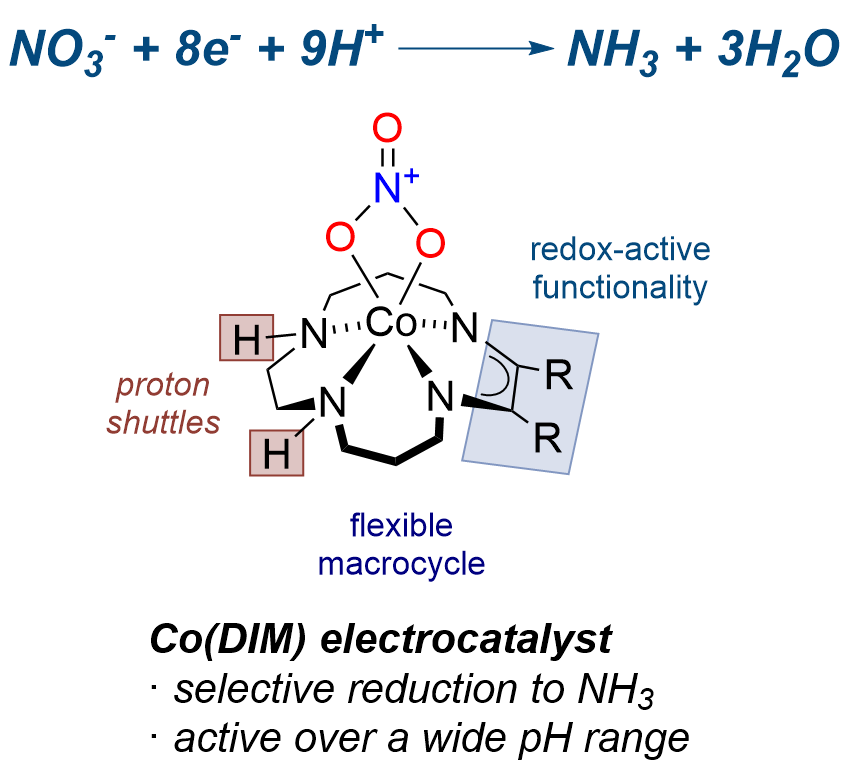
Mechanistic studies into the catalytic activity of the macrocycle complex [Co(DIM)Br2]+ have provided insights into the structural elements required for electrocatalytic activity. We are testing these ideas in the design of molecular complexes and surface-functionalized electrodes electrocatalytic reduction of aqueous nitrate.
Collaborative Research
Our expertise in the design and synthesis of transition metal complexes plays a key role in a number of collaborative research projects. This includes the synthesis complexes featuring metals in unusual coordination environments and/or with strong magnetic coupling, as well as model compounds for systematic interrogation of proton-coupled electron transfer.
We have been funded over the years by a number of federal and private agencies. Our current research projects are funded by the DOE and NSF.




